Implication to 13C, 18O, and clumped 13C18O isotope composition in DIC and calcite
DOI:
https://doi.org/10.3986/ac.v48i1.7208Keywords:
isotope, clumped isotope, speleothem, calcite, paleo- thermometerAbstract
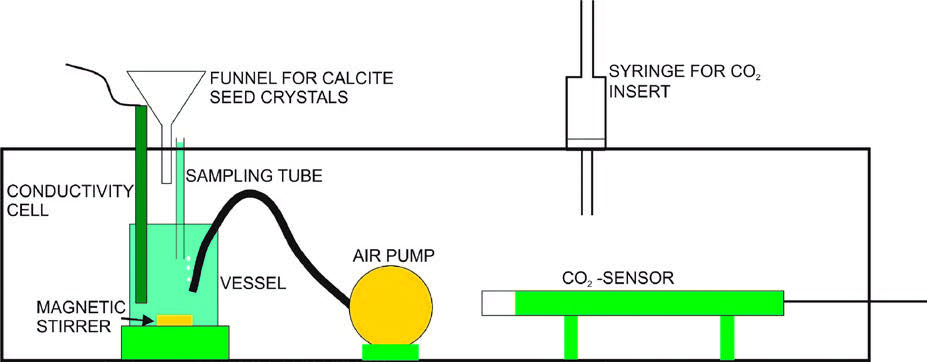
Outgassing of CO2 from thin water layers of a solution of CaCO3 in an H2O -CO2 system plays a crucial role in the precipitation of calcite. Understanding the process of outgassing of CO2 during precipitation of calcite to the surface of stalagmites is important for the interpretation of isotope signals in the calcite deposited to the speleothem. There is, however, some confusion in the literature about the physics and chemistry of this process. Indistinct terms like forced, enhanced, rapid, intense, slow, increased, equilibrium and progressive outgassing are used widely in the literature to explain the impact on isotope composition of the calcite deposited. It is shown that in all the variety of conditions occurring in nature only two distinct processes of outgassing exist. 1. Diffusion controlled outgassing: In the first step, whenever a thin water layer of calcareous solution is present, either on the cave wall or on the surface of a stalagmite, molecular CO2 escapes within several seconds by physical diffusion and after about 40 seconds pH and DIC in the solution achieve chemical equilibrium with respect to the CO2 in the cave atmosphere. 2.) Controlled by precipitation: In the second step this supersaturated solution precipitates calcite, whereby for each unit CaCO3 deposited one molecule of CO2 is generated and escapes from the solution by molecular diffusion. This precipitation controlled outgassing is active during precipitation only. All variations of outgassing mentioned in the literature can be explained by one of these two types of outgassing. Furthermore it is shown that the first step of outgassing driven by diffusion has no influence on the isotope composition of the HCO3 - reservoir in the solution and consequently on that of calcite precipitated from it. The isotope composition of HCO3 for 13C as well as for 18O solely is determined by the second step of precipitation controlled outgassing. An experiment is presented proving that the amount of CO2 escaping from the solution during precipitation of calcite at any time is equal to the amount of calcite precipitated. The results are used for a critical application to the Δ47 clumped isotope thermometer that explains why in most stalagmites the calcite is not a good candidate to obtain correct temperatures at the time of its deposition.
Key words: isotope, clumped isotope, speleothem, calcite, paleo- thermometer.
Fizika in kemija razplinjanja CO2 pri odlaganju sige, s posebnim ozirom na signale izotopa 18O in izotopskega skupka 13C18O v raztopljenem organskem ogljiku in kalcitu
Razplinjanje CO2 iz tanke plasti raztopine sistema CaCO3 in H2O–CO2 je pomembno za izločanje kalcita in interpretacijo izotopskih signalov v odloženem kalcitu. V literaturi je precejšnja zmeda pri obravnavanju fizike in kemije procesa razplinjanja, saj raziskovalci uporabljajo različne izraze, kot so prisiljeno, poudarjeno, počasno, povečano in progresivno razplinjanje. V članku pokažem, da sta pri vseh različnih razmerah v naravi bistvena le dva procesa razplinjanja. 1) Difuzijsko razplinjanje: v prvem koraku molekularni CO2 v nekaj sekundah z difuzijo preide iz tanke plati vode, ki polzi ali po jamski steni ali po površini sige. Po približno 40 sekundah pH in raztopljeni organski ogljik v raztopini dosežeta ravnovesje z atmosferskim CO2. 2) Razplinjanje pri izločanju: v drugem koraku prenasičena raztopina izloča kalcit, pri čemer se za vsako odloženo molekulo CaCO3 iz raztopine sprosti molekula CO2, ki potem z difuzijo uide v jamsko atmosfero. S tema procesoma lahko pojasnimo vse druge načine razplinjanja, ki jih omenja literatura. Nato pokažem, da CO2, ki se razplini v prvem koraku, ne vpliva na izotopsko sestavo zaloge HCO3- v raztopini in zato tudi v izločenem kalcitu. Izotopska sestava HCO3- je tako za 13C in za 18O povsem določena z razplinjanjem med izločanjem kalcita. Ujemanje količine razplinjenega CO2 in izločenega kalcita pokažem tudi s poskusom. Rezultati omogočajo kritično obravnavo uporabe termometra na osnovi izotopskega skupka Δ47 in pojasnjujejo, zakaj kalcit ni primeren za določanje temperature v času izločanja.
Ključne besede: izotop, izotopski skupek, siga, kalcit, paleo temperatura.
Downloads
Downloads
Published
How to Cite
Issue
Section
License
Authors guarantee that the work is their own original creation and does not infringe any statutory or common-law copyright or any proprietary right of any third party. In case of claims by third parties, authors commit their self to defend the interests of the publisher, and shall cover any potential costs.
More in: Submission chapter




