Hidrogeokemična in izotopska karakterizacija ter ocena hidravličnega delovanja kompleksnega kraškega območja Izeh, provinca Khuzestan, jugozahodni Iran
DOI:
https://doi.org/10.3986/ac.v52i1.10687Ključne besede:
Izeh, kemijski izotopi, kras, hidravlična povezanostPovzetek
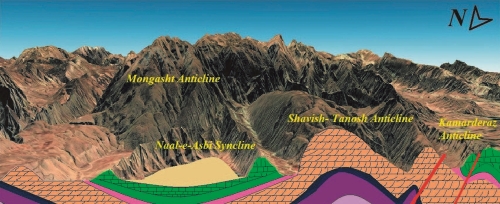
Ustrezno gospodarjenje z vodnimi viri temelji na prepoznavanju in vrednotenju dejavnikov, ki vplivajo na količino in kakovost vodnih virov. Apnenčasto-dolomitni formaciji Ilam-Sarvak (zgornja kreda) in Asmari (oligocen do miocen) v strukturnem pasu Zagros sta oblikovali obetaven kraški vodonosnik. V tej študiji je bil hidravlični odnos med kraškimi strukturami ozemlja Izeh, ki je na severovzhodu province Khuzestan, ocenjen z uporabo hidrogeokemičnih in izotopskih analiz vode v izvirih in vodnjakih. Rezultati so omogočili razumevanje komponent, ki vplivajo na napajanje vodnih virov. Zbrani so bili vzorci iz kraških izvirov in vodnjakov antiklinal Mongašt, Šaviš-Tanoš, Kamarderaz, sinklinale Naal-e-Asbi (podkev) in meteorne vode, da bi razumeli hidrokemično in izotopsko karakterizacijo ter hidrogeološko in hidravlično delovanje kraškega sistema Izeh. Za določitev koncentracij glavnih in drugotnih ionov ter razmerja izotopov δ18O in δ2H so bili analizirani vzorci meteorne in podzemne vode. Vsebnosti izotopov so bile v razponu od ‒31,6 do ‒2,9 ‰ za δ2H in od ‒6,32 do ‒1,87 ‰ za δ18O, vrednosti devterijevega presežka pa so bile visoke in pozitivne. Analize vsebnosti izotopov v vzorcih vode iz izvirov in vodnjakov v regiji kažejo na tri skupine vodnih virov. Prva skupina, ki je povezana z izviri antiklinale Mongašt, ima nižje izotopske vrednosti, kar kaže, da se napaja s padavinami na višjih nadmorskih višinah in s taljenjem snega. Izotopska vrednost druge skupine je bogatejša od prve skupine, kar kaže na napajanje s padavinami in na mešanje podzemnih vod (primera sinklinale Naal-e-Asbi in antiklinale Šaviš-Tanoš). Najvišja vrednost v tretji skupini (vzorci antiklinale Kamarderaz) je posledica delno izhlapevanja in daljše razdalje med mestoma napajanja in iztekanja ter delno difuzijskega sistema. Trend padanja Sr2+ in naraščanja Ba2+ v vzorcih v formacijah dolomitnih apnencev (antiklinali Šaviš-Tanoš in Mongašt) v primerjavi z vzorci vode antiklinale Kamarderaz in sinklinale Naal-e-Asbi kaže na možnost, da se kraški vodonosniki napajajo iz območja antiklinale Mongašt, in na hidravlični odnos med temi strukturami. Devterijev presežek in δ18O kažeta linearni trend, ki ponazarja učinek višinske razlike na vsebnost izotopov in vire napajanja. Večje in manjše spremembe v koncentraciji ionov, vsebnosti izotopov v podzemni vodi in razmerju med TDS in δ18O ter devterijevim presežkom in δ18O kažejo na mešanje in napajanje kraških vodonosnikov (vodonosniki Šaviš-Tanoš, Kamarderaz in Naal-e-Asbi) s kraškega vodonosnika Mongašt in na njihovo hidravlično povezanost.
Prenosi
Literatura
Alavi, N. M., 1996: Tectonic of the Zagros, organic belt of Iran, new data and interpretation. Tectonophysics 299:211–238.
Alemayehu, T., Leis, A. & Dietzel, M, 2020: Environmental isotope and hydrochemical characteristics of groundwater in central portion of Mekelle sedimentary outlier, northern Ethiopia. Journal of African Earth Sciences, 171, [103953]. https://doi.org/10.1016/j.jafrearsci.2020.103953.
Alfarrah, N. & Walraevens, K, 2018: Groundwater overexploitation and seawater intrusion in coastal areas of arid and semi-arid regions Water (Switzerland), 10.3390/w10020143.
Ashjari, J. & Raeisi, E, 2006: Anticline structure influences on regional flow, Zagros, Iran: Journal of Cave and Karst studies, 68 (3), 119-127.
Badaruddin, S. Werner, A.D. & Morgan, L.K, 2017: Characteristics of active seawater intrusion J. Hydrol, 10.1016/j.jhydrol.2017.04.031.
Bagheri, F. Karami, Gh.H. Bagheri, R. Griffioen, J. Eggenkamp, H. & Jafari, H, 2021: Geochemical and multi-isotopes (δ18O, δ2H, δ13C, 3H and δ37Cl) evidences to karst development and flow directions in transboundary aquifer, Northeast of Iran. Applied Geochemistry 132 (2021) 105071. Doi.org/10.1016/j.apgeochem.2021.105071.
Bajjali, W., 2006: Recharge mechanism and hydrochemistry evaluation of groundwater in the Nuaimeh area, Jordan, using environmental isotope techniques. Hydrogeol J 14(1–2):180–191.
Bhat, N.A. & Jeelani, G. H, 2015: Delineation of the recharge areas and distinguishing the sources of karst springs in Bringi watershed, Kashmir Himalayas using hydrochemistry and environmental isotopes. J Earth Syst Sci 124(8):1667–1676. https://doi.org/10.1007/ s12040-015-0629-y.
Blasch, K.W. & Bryson, J.R, 2007: Distinguishing sources of ground water recharge by using delta 2H and delta18O. Ground Water 45(3):294– 308. https://doi.org/10.1111/j.1745-6584.2006.00289.x.
Bourke, S. Hammond, M. & Clohessy, S, 2015: Perth shallow groundwater systems investigation: North Lake. Retrieved from http://www. water.wa.gov.au/Publication Store/first/91255.pdf.
Celle-Jeanton, H. Travy, Y. & Blavoux, B, 2001: isotopic typology of the precipitation in the Western Mediterranean region at three different time scales. Geophys Res Lett 28:1215–1218.
Chen, J. S. Li, L. Wang, J.Y. Barry, D. A. Sheng, X. F. Gu, W. Z. Zhao, X. & Chen, L, 2004: Water resources: groundwater maintains dune landscape, Nature, 432, 459–460, https://doi.org/10.1038/432459a.
Chen, W. Li, H. Hou, E. Wang S, Wang G, Panahi M, Li T, Peng T, Guo C. & Niu, C, 2018: Gis-based groundwater potential analysis using novel ensemble weights-of-evidence with logistic regression and functional tree models. Sci. Total Environ. 634, 853–867.
Chihi, H. Marsily, G. Belayouni, H. & Yahyaoui, H, 2015: Relationship between tectonic structures and hydrogeochemical compartmentalization in aquifers: example of the Jeffara de Medenine system, south-east Tunisia. J Hydrol Reg Stud 4:410–430.
Ciner, F. Sunkari, E. D. & Senbas, B.A. 2021: Geochemical and Multivariate Statistical Evaluation of Trace Elements in Groundwater of Nigde Municipality, South-Central Turkey: Implications for Arsenic Contamination and Human Health Risks Assessment. Arch Environ Contam Toxicol 80, 164–182 (2021). https://doi.org/10.1007/s00244-020-00759-2.
Clark, I.D. & Fritz, P, 1997: Environmental Isotopes in Hydrogeology; Lewis Publishers: Boca Raton, FL, USA, p. 352.
Connor, JA. Paquette, S. Mchugh, T. Gie, E. Hemingway, M. & Bianchi, G, 2017: Application of natural resource valuation concepts for development of sustainable remediation plans for groundwater. J Environ Manag 204:721.
Coplen, T. B., 1993: Uses of Environmental Isotopes, in: Alley, W. M. (ed.), Regional Water Quality, Van Nostrand Reinhold, pp. 227–254.
Dansgaard, W. White, J.W. & Johnsen, S.J, 1989: The abrupt termination of the Younger Dryas climate event. Nature 339: 532–534.
Dimitriou, E. & Tsintza, P, 2015: Hydrogeologic Investigations in Western Crete by Using Isotopic Analyses and GIS Techniques. Journal of Water Resource and Protection, 7, 923-937. doi: 10.4236/jwarp.2015.712076.
Doctor, DH. E. Calvin Alexander, Jr. Petric, M. & Kogovsek, J, 2006: Quantification of karst aquifer discharge components during storm events through end-member mixing analysis using natural chemistry and stable isotopes as tracers. Hydrogeology Journal 14(7):1171-1191 DOI:10.1007/s10040-006-0031-6.
Doveri, M. Menichini, M. & Cerrina Feroni, A, 2013: Stable water isotopes as fundamental tool in karst aquifer studies: some results from isotopic applications in the Apuan Alps carbonatic complexes (NW Tuscany). Ital J Eng Geol Environ 1:33–50.
Edmunds, W.M. Ma, J.Z. Aeschbach-Hertig, W. Kipfer, R. & Darbyshire, D.P.F, 2006: Groundwater recharge history and hydrogeochemical evolution in the Minqin Basin, North West China. Appl Geochem 21(12):2148–2170.
Edwards, T.W.D. Wolfe, B.B. Gibson, J.J. & Hammarlund, D. 2004: Use of water isotope tracers in high-latitude hydrology and paleohydrology. In: Pienitz, R., Douglas, M.S.V., Smol, J.P. (Eds.), Long-term Environmental Change in Arctic and Antarctic Lakes. Springer, Dordretch, The Netherlands, 187–207 pp.
Emblanch, C. Zuppi, G.M. Mudry, J. Blavoux, B. & Batiot, C, 2003: Carbon 13 of TDIC to quantify the role of the unsaturated zone: the example of the Vaucluse karst systems (Southeastern France). J Hydrol 279(1):262–274.
Fairchild, I.J. Borsato, A. Tooth, A.F. Frisia, S. Hawkes-worth, C.J. Huang, y. McDermott, F. & Spiro B, 2000: Controls on trace element (Sr–Mg) composi-tions of carbonate cave waters: implications for spe-leothem climatic records.- Chemical Geology, 166, 255–269.
Farid, I. Abbas, MHH. & Bassouny, M, 2020: Indirect impacts of irrigation with low quality water on the environmental safety. Egypt J Soil Sci. https:// doi. org/ 10. 21608/ ejss. 2019. 15434. 1294.
Florea, L.J. & McGee, D.K, 2010: Isotopic and geochemical variability within shallow groundwater beneath a hardwood hammock and surface water in an adjoining slough (Everglades National Park, Florida, USA). Isot. Environ. Health Stud, 46, 190–209.
Ford, D.C. & P.W. Williams, 1989: Karst Geomorphology and Hydrology. Unwin Hyman, London.
Ford, D.C. & P.W. Williams, 2007: Karst Geomorphology and Hydrology. John Wiley and Sons.
Frohlich, K. & Gibson, J.J, 2015: Aggarwal, P. Deuterium Excess in Precipitation and Its Climatological Significance. Available online: http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/ 34/017/34017972.pdf.
Gates, J. B. Edmunds, W. M. Darling, W. G. Pang, J. Ma, Z. & Young, A. A, 2008: Conceptual model of recharge to southeastern Badain Jaran Desert groundwater and lakes from environmental tracers, Appl. Geochem., 23(12), 3519– 3534.
Gibbs, R.J., 1970: Mechanisms controlling world water chemistry. Science 17:1088–1090.
Goldscheider, N. & Andreo, B, 2007: The geological and geomorphological framework. In: Goldscheider, N., Drew, D. (Eds.). Methods in karst hydrogeology. International Contribution to Hydrogeology, IAH, vol 26. Taylor and Francis/Balkema, London, 9–23.
Haldar. K/ Kujawa-Roeleveld, K. & Dey, P, 2020: Spatio-temporal variations in chemical-physical water quality parameters influencing water reuse for irrigated agriculture in tropical urbanized deltas. Sci Total Environ 708:134559. https://doi.o rg/1 0.1 016/j. scito tenv. 2019. 134559.
Hatipoglu-Bagci, Z. & Sazan, M.S, 2014: Characteristics of karst springs in Aydıncık (Mersin, Turkey), based on recession curves and hydrochemical and isotopic parameters. Quarterly Journal of Engineering Geology and Hydrogeology (2014),47(1):89.
Helena, B. Pardo, R. Vega, M. Barrado, E. Fernandez, J.M. & Fernandez, L, 2000: Temporal evolution of groundwater composition in an alluvial (Pisuerga river, Spain) by principal component analysis. Water Research 34, 807–816.
Heydarizad, M. Minaei, F. Eskandari Mayvan, J. Mofidi, A. & Minaei ,M, 2021: Spatial distribution of stable isotopes (18O and 2H) in precipitation and groundwater in Iran, Isotopes in Environmental and Health Studies, 57:4, 400-419, DOI: 10.1080/10256016.2021.1924167.
Hounslow, A.W., 1995: Water Quality Data: Analysis and Interpretation, CRC Lewis Publishers, Boca Raton, FL (1995) 86–87.
Jebreen, H. Banning, A. Wohnlich, S. Niedermayr, A. Ghanem, M. & Wisotzky, F, 2018: The Influence of Karst Aquifer Mineralogy and Geochemistry on Groundwater Characteristics: West Bank, Palestine. Jornal Water 2018, 10, 1829; doi:10.3390/w10121829.
Jiang, L. Sui, M. Fan, Y. Su, H. Xue, Y. & Zhong, Sh, 2021: Micro-gas column assisted laser induced breakdown spectroscopy (MGC-LIBS): A metal elements detection method for bulk water in-situ analysis. Spectrochimica Acta Part B: Atomic Spectroscopy. Volume 177, March 2021, 106065.
Johnsen, S.J. Dansgaard, W. &White, J.W.C, 1989: The origin of Arctic precipitation under present and glacial conditions. Tellus B 41: 452–468.
Jouzel, J. Stievenard, M, S.J. Johnsen, A. Landais, V. Masson-Delmotte, A. Sveinbjornsdottir, F. Vimeux, U. Grafenstein, von. & White, J.W.C, 2007: The GRIP deuteriumexcess record. Quaternary Science Reviews 26, no. 1–2: 1–17.
Kalantari, N. Pawar, N.J. & Keshavarzi, M.R, 2009: Water resource management in the intermountain Izeh Plain, Southwest of Iran. J. Mt. Sci. 6, 25–41, https://doi.org/10.1007/s11629-009-0212-6.
Krienen, L. Heuser, M. & Hobig, N, 2017: Hydrogeological and hydrochemical characterization of two karstic discharge areas in San Luis Potosí, Mexico. Environ Earth Sci 76, 825. https://doi.org/10.1007/s12665-017-7166-8.
Langelier, WF. & Ludwig, H.F, 1942: Graphic method for indicating the mineral character of natural water. J Am Water Works Assoc 34:335–352.
Lewinska-Preis, L. Szram, E. & Fabiańska, M.J, 2021: Selected ions and major and trace elements as contaminants in coal-waste dump water from the Lower and Upper Silesian Coal Basins (Poland). Int J Coal Sci Technol 8, 790–814. https://doi.org/10.1007/s40789-021-00421-9.
Li, X. Han, G. Liu, M. Liu, J. Zhang, Q. & Qu, R, 2021: Potassium and its isotope behaviour during chemical weathering in a tropical catchment affected by evaporite dissolution, Geochimica et Cosmochimica Acta https://doi.org/10.1016/j.gca.2021.10.009.
Liu, P. Yang, M. &Sun, Y, 2019: Hydro-geochemical processes of the deep Ordovician groundwater in a coal mining area, Xuzhou, China. Hydrogeol. J. https://doi.org/ 10.1007/s10040-019-01991-4.
Ma, J.Z. He, J.H. Qi, S. Zhu, G.F. Zhao, W. Edmunds, W.M. & Zhaom, Y.P, 2013: Groundwater recharge and evolution in the Dunhuang Basin, northwestern China. Appl Geochem 28:19–31.
Mahlknecht, J. Garfias-Solis, J. Aravena, R. & Tesch, R, 2006: Geochemical and isotopic investigations on groundwater residence time and flow in the Independence Basin, Mexico. J Hydrol 324:283–300.
Makhloufi, Y. Rusillon, E. Brentini, M. Moscariello, A. Meyer, M. & Samankassou, E, 2018: Dolomitization of the Upper Jurassic carbonate rocks in the Geneva Basin, Switzerland and France. Swiss Journal of Geosciences 111:475–500 https://doi.org/10.1007/s00015-018-0311-x.
Marfia, A.M. Krishnamurthy, R.V. Atekwana, E.A. & Panton, W.F, 2004: Isotopic and geochemical evolution of groundwater and surface waters in a karst-dominated geological setting: a case study from Belize, Central America. Appl Geochem 19:937–946.https://doi.org/10.1016/j.apgeochem. 2003. 10.013.
Masson-Delmotte, V. Jouzel, J. Landais, A. Stievenard, M. Johnsen, S.J. White, J.W.C. Werner, M. Sveinbjornsdottir, A. & Fuhrer ,K, 2005: GRIP deuterium excess reveals rapid and orbital-scale changes in Greenland moisture origin. Science 309, no. 1: 118–121.
Mazor, E, 2004: Chemical and isotopic groundwater hydrology, 3rd edn. Weizmann Institute of Science Rehovot, New York 465p.
Milanovic, P. T., 1981: Karst hydrogeology Water Resources publications, 434 p.
Mokadem, N. Dennis, R. & Dennis, I, 2021: Hydrochemical and stable isotope data of water in karst aquifers during normal flow in South Africa, Environmental Earth Sciences 80:519 https://doi.org/10.1007/s12665-021-09845-7.
Moral, F. Cruz-Sanjulian, J.J. & Olias, M, 2008: Geochemical evolution of groundwater in the carbonate aquifers of Sierra de Segura (Betic Cordillera, southern Spain). Journal of Hydrology (2008) 360, 281– 296.
Morsy, KM. Morsy, AM. & Hassan, AE, 2018: Groundwater sustainability: opportunity out of threat. Groundw Sustain Dev 7:277–285.
Murillo, R.S. Brooks, E. Elliot, J.W. & Bolla, J, 2015: Isotope hydrology and baseflow geochemistry in natural and human-altered watersheds in the inland Pacific Northwest, USA. Isot Environ Health Stud 51:231–254. https://doi.org/10.1080/10256016.2015.1008468.
Nader, FH. Swennen, R. & Ottenburgs, R, 2003: Karst-meteoric dedolomitization in Jurassic carbonates, Lebanon. Geologica Belgica 6:3–23.
Narany, S.T. Ramli, M.F. Aris, A.Z. Sulaiman, W.N.A. Juahir, H. & Fakharian, K, 2014: Identification of the hydrogeochemical processes in groundwater using classic integrated geochemical methods and geostatistical techniques in Amol-Babol plain, Iran. Sci World J 2014:1–15. https://doi.org/10.1155/2014/419058.
Negrel, Ph. & Petelet-Giraud, E, 2005: Strontium isotopes as tracers of groundwaterinduced floods: the Somme case study (France). J. Hydrol. 305, 99–119.
Piper, A.M., 1944: A graphic procedure in the geochemical interpretation of water analyses. Trans Am Geophys Union 6:914–923.
Porowski, A., 2004: Isotopic evidence of the origin of mineralized waters from the Central Carpathian Synclinorium, SE Poland. Environmental Earth Sciences, 46(5):661-669. DOI:10.1007/s00254-004-1005-4.
Pracny, P. Faimon, J. & Vsiansky, D, 2017: Evolution of Mg/Ca Ratios During Limestone Dissolution Under Epikarstic Conditions. Aquat Geochem 23, 119–139. https://doi.org/10.1007/s10498-017-9313-y.
Pratama, A. D. Dwiputra, D. S. Nurkholis, A. Haryono, E. Cahyadi, A. & Fauzan, R, 2021: Factors Affecting Hydrochemistry of Karst Springs and their Relationship to Aquifer Development. Environ. Process. 8, 1379–1413 (2021). https://doi.org/10.1007/s40710-021-00547-7.
Rademacher, L.K. Clark, J.F. & Boles, J.R, 2003: Groundwater residence times and flow paths in fractured rock determined using environmental tracers in the Mission tunnel: Santa Barbara County, California, USA. Environ Geol 43:557–567.
Rajmohan, N. & Elango, L, 2004: Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar river basins, southern India. Environ Geol 46(1): 47–61. https://doi.org/10.1007/s00254-004-1012-5.
Rehman, F. Cheema, T. & Azeem, T, 2019: Groundwater quality of sargodha city and its suitability for domestic and irrigation purpose. Fresenius Environ Bull 28(11):7695–7700.
Ren, M. & Jones, B, 2017: Spatial variations in the stoichiometry and geochemistry of Miocene dolomite from Grand Cay-man: implications for the origin of island dolostone. Sedi-mentary Geology 348, 69–93.
Rosenbaum, J., Sheppard, S.M.F. 1986.
Rodgers, P. Soulsby, C. Waldron, S. & Tetzlaff, D, 2005: Using stable isotope tracers to assess hydrological flow paths, residence times and landscape influences in a nested mesoscale catchment. Hydrol Earth Syst Sci 9:139–155.
Ryu, J.S. Lee, K.S. & Chang, H.W, 2007: Hydrogeochemical and isotopic investigations of the Han River basin, South Korea. J Hydrol 345:50–60.
Scanlon, R. Healy, W. & Cook, G, 2002: Choosing appropriate techniques for quantifying groundwater recharge. Hydrogeol. J., 10, 18–39.
Setiawan, T. Yoseph, C.S.S.B. Alam, S. Haryono, E. & Hendarmawan, Ir, 2020: Hydrochemical and environmental isotopes analysis for characterizing a complex karst hydrogeological system of Watuputih area, Rembang, Central Java, Indonesia, Hydrogeology Journal . Issue 5/2020.
Singh, M. Kumar, S. Kumar, B. Singh, S. & Singh, I.B, 2013: Investigation on the hydrodynamics of Ganga alluvial plain using environmental isotopes: a case study of the Gomati River basin, northern India. Hydrogeol J 21:687–700.
Slabe, T. & Liu, H, 2009: Significant subsoil rock forms. In: Ginés A, Knez M, Slabe T, Dreybrodt W (eds) Karst rock features, karren sculpturing. Carsologica 9, ZRC Publishing, Ljubljana, pp 123−137.
Srivastava, S.K. & Ramanathan, A.L, 2008: Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environmental Geology 53.1509–1528.
Stocklin, J., 1974: Evolution of the continental margins bounding a former Southern Tethys. In: Geology of continental margins. Springer, Berlin, pp 873–887, BIBL. 2P, 5Illus. U.N. Geological Survey Institute.
Sun, Z. Ma, R. Wang, Y. Ma, T. & Liu, Y, 2016: Using isotopic, hydrogeochemical-tracer and temperature data to characterize recharge and flow paths in a complex karst groundwater flow system in northern China. Hydrogeol J 24:1393–1412. https://doi.org/10.1007/s10040-016-1390-2.
Tang, L. Zhao, Y. Zhang, Sh. Sun, T. KaiHu, L. Ming, X. Sheng, Y. & Zeng, T, 2021: Origin and evolution of a porphyry-breccia system: Evidence from zircon U-Pb, molybdenite Re-Os geochronology, in situ sulfur isotope and trace elements of the Qiyugou deposit, China. Gondwana Research, Volume 89, January 2021, Pages 88-104.
Tian, L. Gao, Y. Yang, G. Schwartz, B. Cai, B. Lei, G. Shi, G. Ray, Ch. Sok, S. Martinez, E. Li, Y. & Wu, H, 2021: The evolution of hydrochemical and isotopic signatures from precipitation, surface water to groundwater in a typical karst watershed, Central Texas, USA, Isotopes in Environmental and Health Studies, 57:5, 492-515, DOI: 10.1080/10256016.2021.1948410.
Tillman, F.D. Oki, D.S. Johnson, A.G. Barberm, L.B. & Beisner, K.R, 2014: Investigation of geochemical indicators to evaluate the connection between inland and coastal groundwater systems near Kaloko-Honoko-hau National Historical Park, Hawai‘i. Appl Geochem 51:278–292.https://doi.org/10.1016/j.apgeochem.2014.10.003.
Valdes, D. Dupont, J.P. Laignel, B. Ogier, S. Leboulanger, T. & Mahler, B.J, 2007: A spatial analysis of structural controls on karst groundwater geochemistry at a regional scale. J Hydrol 340:244–255. https://doi.org/10.1016/j.jhydrol.2007.04.014.
Ventura-Houle, R. Guevara-Mansilla, O. & Requena-Lara, G, 2021: Hydrochemistry, δD and δ18O to explain the distribution of water quality in a karst setting in the semi-arid region of Northeast Mexico. Environ Earth Sci 80, 6 .https://doi.org/10.1007/s12665-020-09310.
Vreca, P. & Kern, Z, 2021: Use of Water Isotopes in Hydrological Processes. Water, 12, 2227.
Wang, Y. Song, X. & Li, B, 2018: temporal variation in groundwater hydrochemistry driven by natural and anthropogenic processes at a reclaimed water irrigation region. Hydrol Res 49(5):1652–1668. https:// doi. Org/ 10. 2166/ nh. 2018. 123
Welhan, J.A., 1987: Stable isotope hydrology. In: Kyser TK (ed) Short Course in Stable isotope geochemistry of low temperaturefluids. Mineralogical Association of Canada, Vol. 13, pp 129—161.
White, W.B., 2015: Chemistry and karst. Acta Carsologica. Vol.44. Iss. 3 p. 349–362. DOI 10.3986/ac.v44i3.1896.
WHO, 2004: International standards of drinking water. World Health organization, Geneva. pp: 55-79.
Yasin, D. & Kargın, M, 2021: Hydrogeochemical and isotopic characteristics of water resources in Cubuk‑Meliksah (Ankara/Turkey). Environmental Earth Science, 80:513 https://doi.org/10.1007/s12665-021-09813-1.
Yuan, R. Wang, S. Wang, V. Song, X. & Tang, C, 2017: Changes in flow and chemistry of groundwater heavily affected by human impacts in the Baiyangdian catchment of the North China Plain Environ. Earth Sci., 76 (2017), p. 19, 10.1007/s12665-017-6918-9.
Zaidi, F.K. Nazzal, Y. Jafri, M.K. Naeem, M. & Ahmed, I, 2015: Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: a case study from northwestern Saudi Arabia. Environ Monit Assess 187(10):607. https://doi.org/10. 1007/s10661-015-4828-4.
Zanchi, A. Zanchetta, S. Berr, F. Mattei, M. Garzanti, E. Molyneux, S. & Sabouri, J, 2009: The Eo-Cimmerian (late? Triassic) orogeny in North Iran. Geol Soc Lond Spec Publ 312(1):31–55. https://doi.1. org/10.1144/SP312.3.
Zhang, W. Li, L. Wang, X. Xing, W. Li, R. Yang, T. & Lv, D, 2020: Role of trace elements in anaerobic digestion of food waste: Process stability, recovery from volatile fatty acid inhibition and microbial community dynamics. Bioresource Technology, Volume 315, November 2020, 123796.
Zhou, J. Zhang, Y. Zhou, A. Liu, C. Cai, H, Liu, & Y, 2016: Application of hydrochemistry and stable isotopes (δ34S, δ18O and δ37Cl) to trace natural and anthropogenic influences on the quality of groundwater in the piedmont region, Shijiazhuang, China. Appl Geochem 71:63–72. https://doi.org/10.1016/j.apgeochem. 2016.05.018.
Prenosi
Objavljeno
Kako citirati
Številka
Rubrike
Licenca

To delo je licencirano pod Creative Commons Priznanje avtorstva 4.0 mednarodno licenco.
Avtorji jamčijo, da je delo njihova avtorska stvaritev, da v njem niso kršene avtorske pravice tretjih oseb ali kake druge pravice. V primeru zahtevkov tretjih oseb se avtorji zavezujejo, da bodo varovali interese založnika ter da bodo povrnili morebitno škodo.
Podrobneje v rubriki: Prispevki




